Article
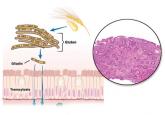
Celiac disease: Managing a multisystem disorder
This autoimmune disorder can cause symptoms that involve not only the gastrointestinal tract but also the skin and bones.
JOSE U. SCHER, MD
Assistant Professor of Medicine, New York University Division of Rheumatology; Director, Arthritis Clinic and Psoriatic Arthritis Center; Director, Microbiome Center for Rheumatology and Autoimmunity (MiCRA), New York University-Langone Hospital for Joint Diseases, New York, NY
ADDRESS: Jose U. Scher, MD, Division of Rheumatology, NYU Hospital for Joint Diseases, 301 East 17th Street, Room 1608, New York, NY 10003; e-mail: Jose.Scher@nyumc.org

To move beyond correlative studies and mechanistically address the possibility of causation, multiple groups have used a gnotobiotic approach, ie, maintaining animals under germ-free conditions and incorporating microbes of interest. This approach is highly relevant in studying whether the bacterial community composition is capable of modulating loss of tolerance to gluten in genetically susceptible hosts. A few notable examples have been published.
In germ-free rats, long-term feeding of gliadin, but not albumin, from birth until 2 months of age induced moderate small-intestinal damage.21 Similarly, germ-free nonobese diabetic-DQ8 mice developed more severe gluten-induced disease than mice with normal intestinal bacteria.22
In small studies, people with celiac disease had fewer Firmicutes and Bifidobacteria and more Proteobacteria, Bacteroides, and E coli
These findings suggest that the normal gut microbiome may have intrinsic beneficial properties capable of reducing the inflammatory effects associated with gluten ingestion. Notably, the specific composition of the intestinal microbiome can define the fate of gluten-induced pathology. Mice colonized with commensal microbiota are indeed protected from gluten-induced pathology, while mice colonized with Proteobacteria spp develop a moderate degree of gluten-induced disease. When Escherichia coli derived from patients with celiac disease is added to commensal colonization, the celiac disease-like phenotype develops.23
Taken together, these studies support the hypothesis that the intestinal microbiome may be another environmental factor involved in the development of celiac disease.
QUESTIONS AND CHALLENGES REMAIN
The results of clinical studies are not necessarily consistent at the taxonomy level. The fields of metagenomics, which investigates all genes and their enzymatic function in a given community, and metabolomics, which identifies bacterial end-products, characterizing their functional capabilities, are still in their infancy and will be required to further investigate functionality of the altered microbiome in celiac disease.
Second, the directionality—the causality or consequences of this dysbiosis—and timing—the moment at which changes occur, ie, after introducing gluten or at the time when symptoms appear—remain elusive, and prospective studies in humans will be essential.
Finally, more mechanistic studies in animal models are needed to dissect the host immune response to dietary gluten and perturbation of intestinal community composition. This may lead to the possibility of future interventions in the form of prebiotics, probiotics, or specific metabolites, complementary to gluten avoidance.
In the meantime, increasing disease awareness and rapid diagnosis and treatment continue to be of utmost importance to address the clinical consequences of celiac disease in both children and adults.
Supported by: Grant No. K23AR064318 from NIAMS to Dr. Scher; The Colton Center for Autoimmunity; The Riley Family Foundation.

This autoimmune disorder can cause symptoms that involve not only the gastrointestinal tract but also the skin and bones.
If you had a serious disease, would you agree to an alternative treatment that is cheap, safe, and effective—but seems disgusting? Would you...
The disease is underrecognized because about half of people who have it do not have the classic gastrointestinal symptoms. Instead, they may...
