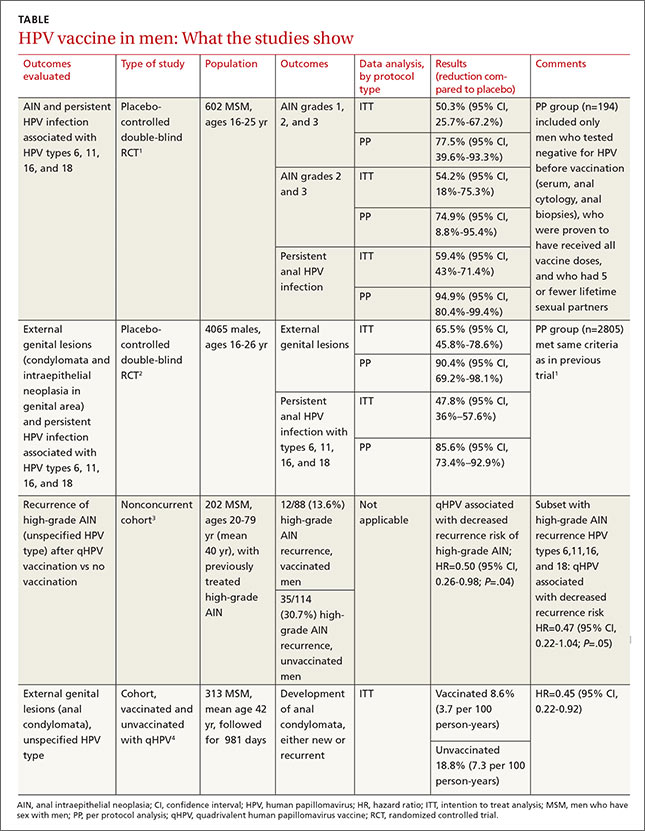
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).
The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5