Case Letter
Psoriasis and Leprosy: A Mystifying Coexistence
Psoriasis and leprosy exist mutually exclusively with only a few cases being reported regarding their coexistence. Among the various forms of...
Sarah M. Sung, MD; Todd T. Kobayashi, MD
Dr. Sung is from Johns Hopkins Hospital, Baltimore, Maryland. Dr. Kobayashi is from the San Antonio Uniformed Services Health Education Consortium, Texas.
The authors report no conflict of interest.
The opinions expressed are those of the authors and do not necessarily reflect the official policy or position of the US Air Force or the US Department of Defense.
Correspondence: Sarah M. Sung, MD, Johns Hopkins Hospital, Department of Dermatology, 1500 Orleans St, Baltimore, MD 21231.

Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14

Psoriasis and leprosy exist mutually exclusively with only a few cases being reported regarding their coexistence. Among the various forms of...
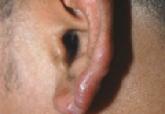
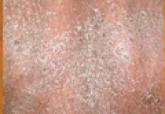
A 37-year-old man presented with pruritic lesions over the arms, legs, face, and back of 4 months’ duration that had been refractory to topical...
Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode...
