Pediatric Dermatology
Cutaneous Side Effects of Chemotherapy in Pediatric Oncology Patients
Pediatric oncology patients can present with various skin lesions related to both primary disease and immunosuppressive treatments. This study...
Anis Miladi, MD; Brian C. Thomas, MD; Knox Beasley, MD; Jon Meyerle, MD
Dr. Miladi is from the Department of Dermatology, Naval Medical Center Portsmouth, Virginia. Dr. Thomas is from Tennessee River Dermatology, Florence, Alabama. Dr. Beasley is from William Beaumont Army Medical Center, El Paso, Texas. Dr. Meyerle is from Uniformed Services University of the Health Sciences, Bethesda.
The authors report no conflict of interest. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Department of Defense, or the US Government. This case report was presented in part at the 69th Annual Meeting of the American Academy of Dermatology; February 4-8, 2011; New Orleans, Louisiana.
Correspondence: Anis Miladi, MD, Department of Dermatology, Naval Medical Center Portsmouth, 620 John Paul Jones Circle, Portsmouth, VA 23708-2197 (anis.miladi@med.navy.mil).

Purpura fulminans is a nonspecific hematologic emergency with high initial mortality, representing a thrombotic occlusion of blood vessels leading to skin necrosis and disseminated intravascular coagulation, and often reported in the setting of sepsis. We report a case of nonfatal purpura fulminans in the context of angioimmunoblastic T-cell lymphoma (AITL).
Practice Points
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
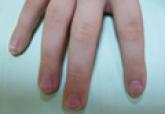
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
| Figure 1. Purpuric patches on the left foot (A). Several days later there were geographic plaques of noninflammatory purpura and ecchymoses with hemorrhagic bullae. There was a sharp demarcation between involved and uninvolved skin (B). |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
Figure 2. Fibrin thrombi filled the lumen of small arteries, arterioles, and capillaries. Red blood cell extravasation with paucicellular subepidermal bulla overlying a degenerative dermis also was seen (A)(H&E, original magnification ×100). A noninflammatory fibrin thrombus occluded a mid dermal vessel (B)(H&E, original magni-fication ×400). |
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4

Pediatric oncology patients can present with various skin lesions related to both primary disease and immunosuppressive treatments. This study...
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus....
