Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
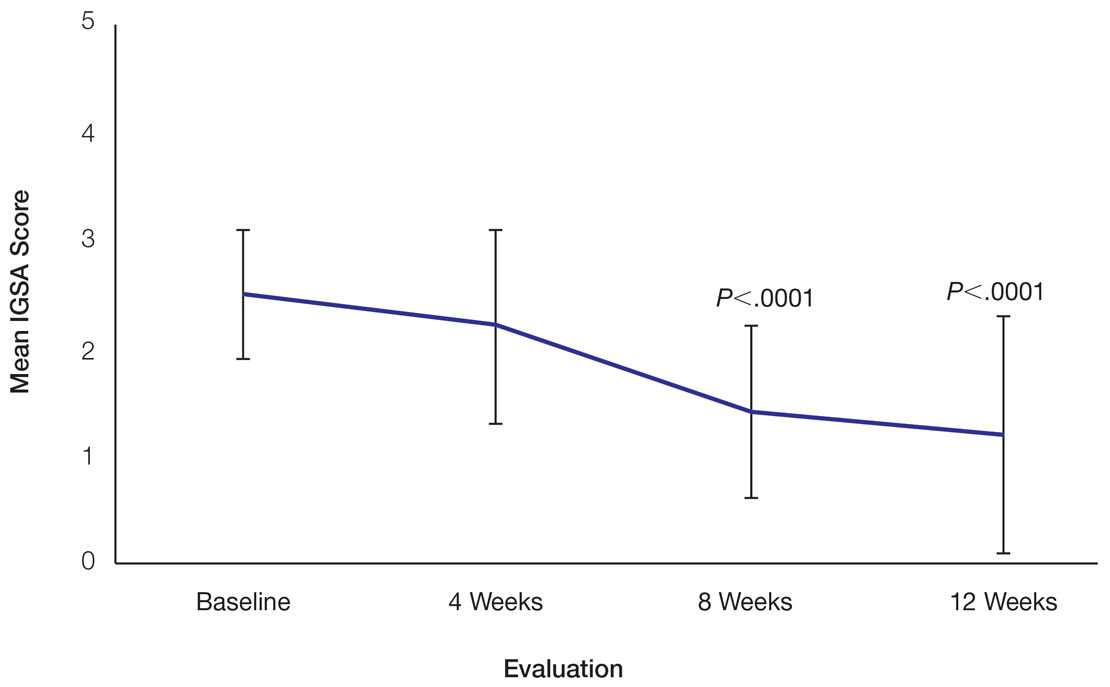
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.
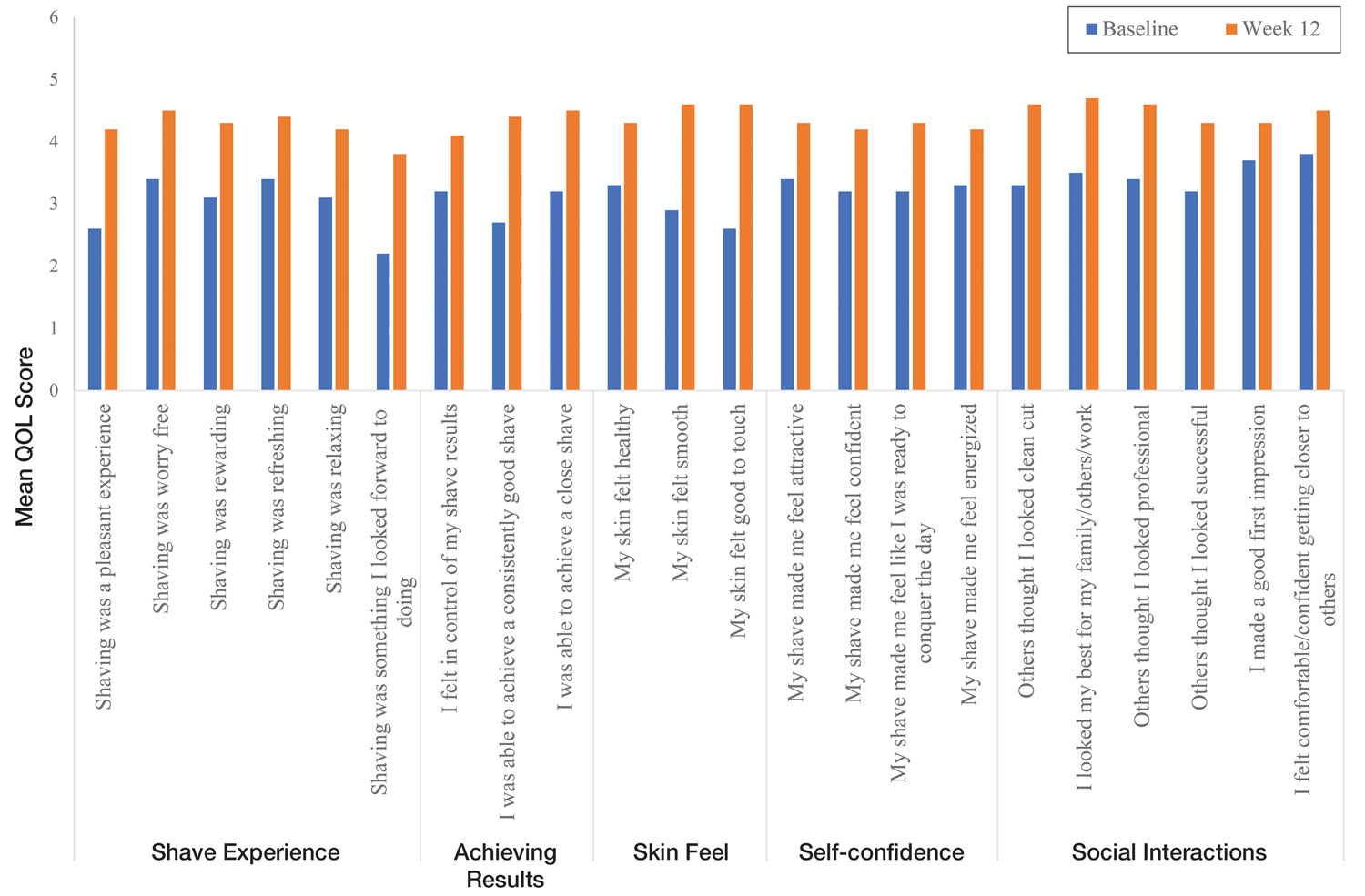
Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.