Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
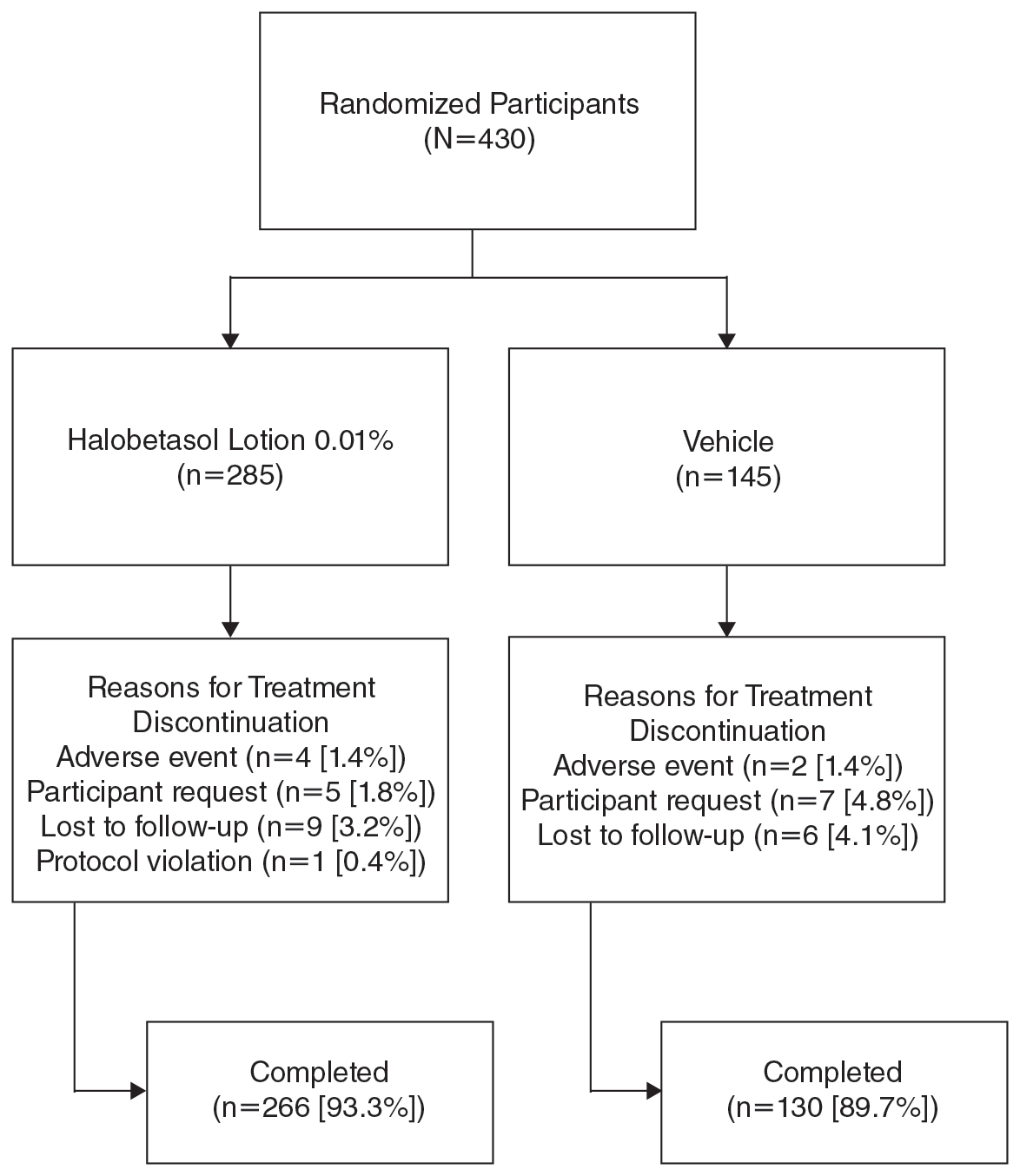
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
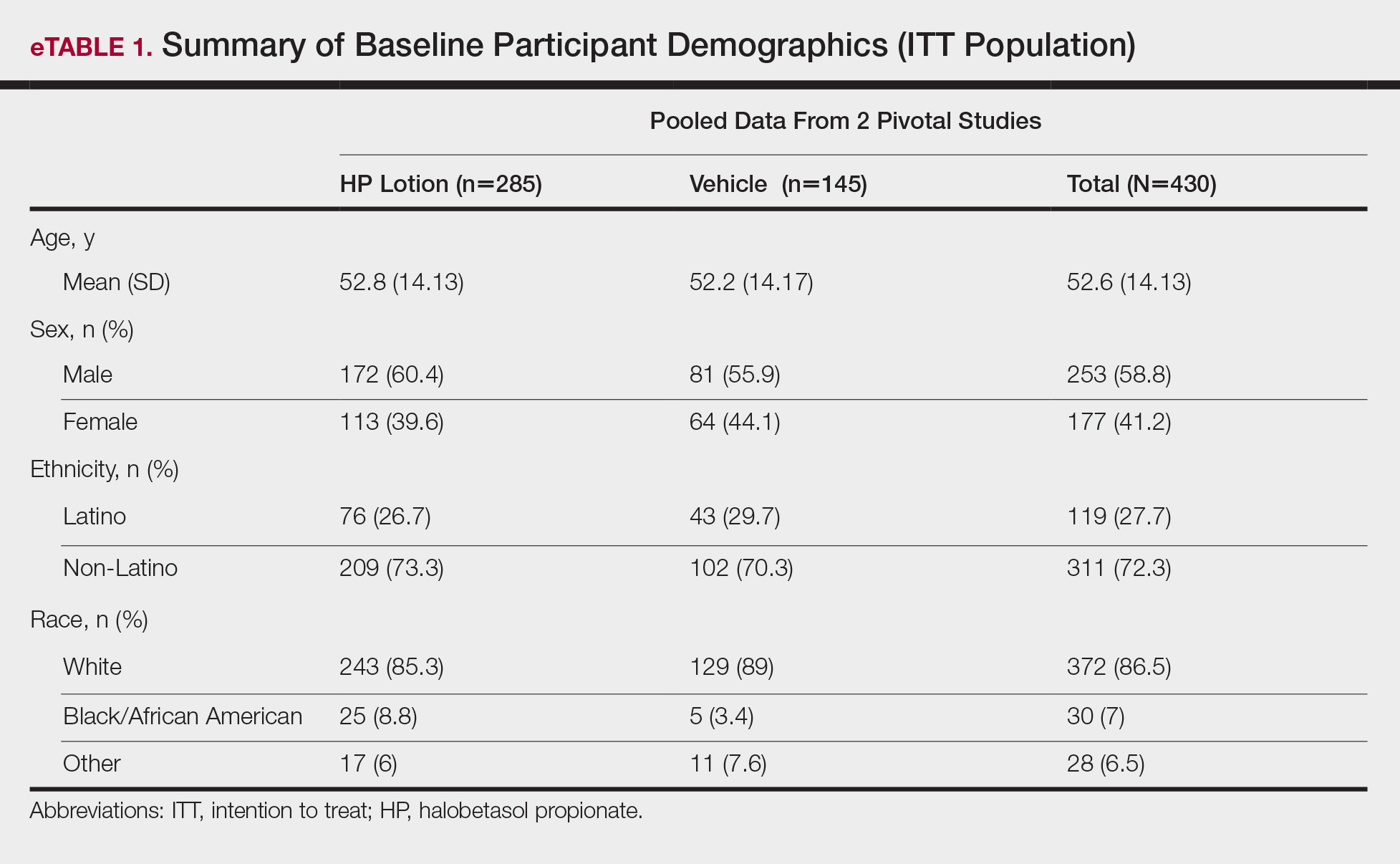
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
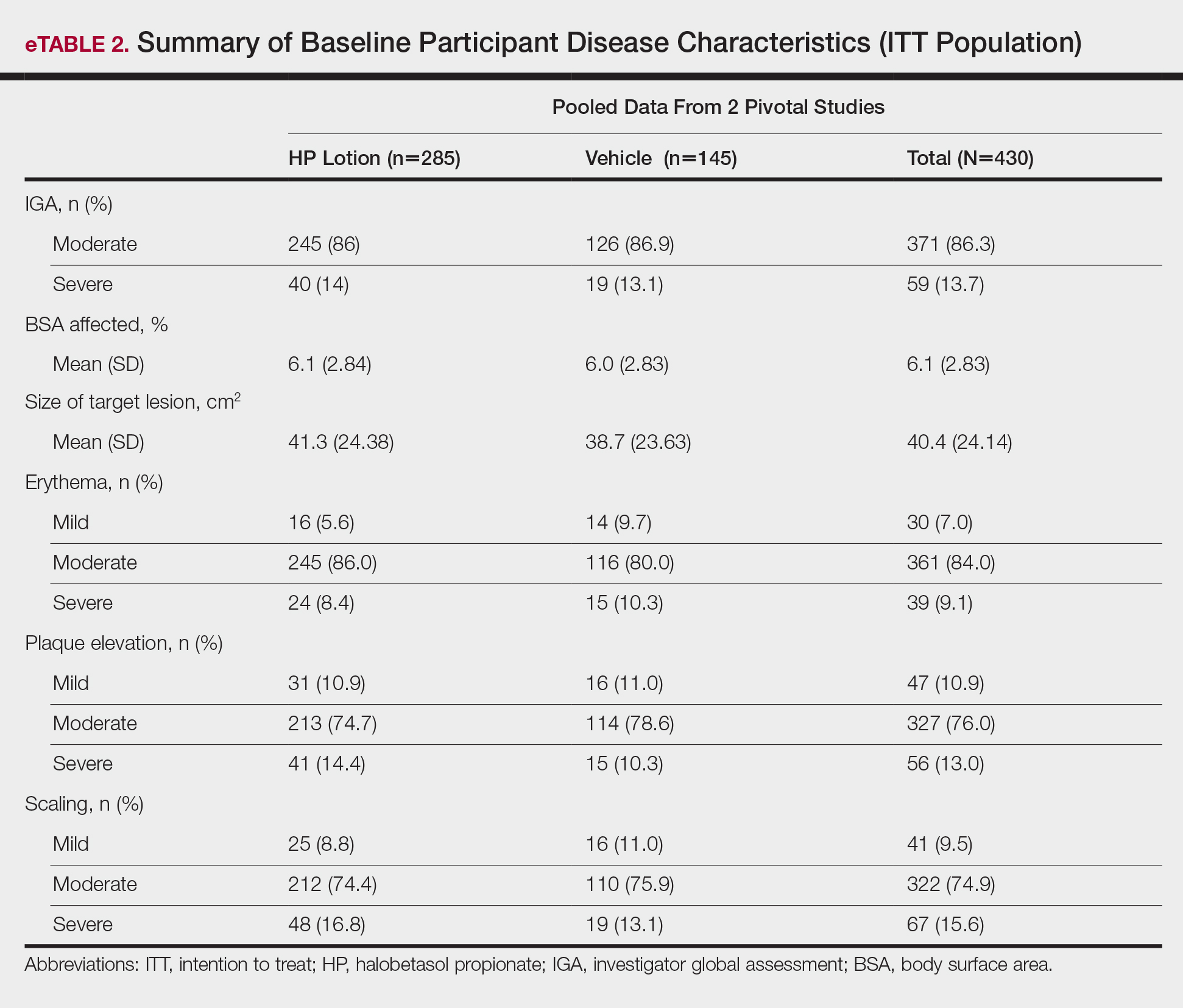
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
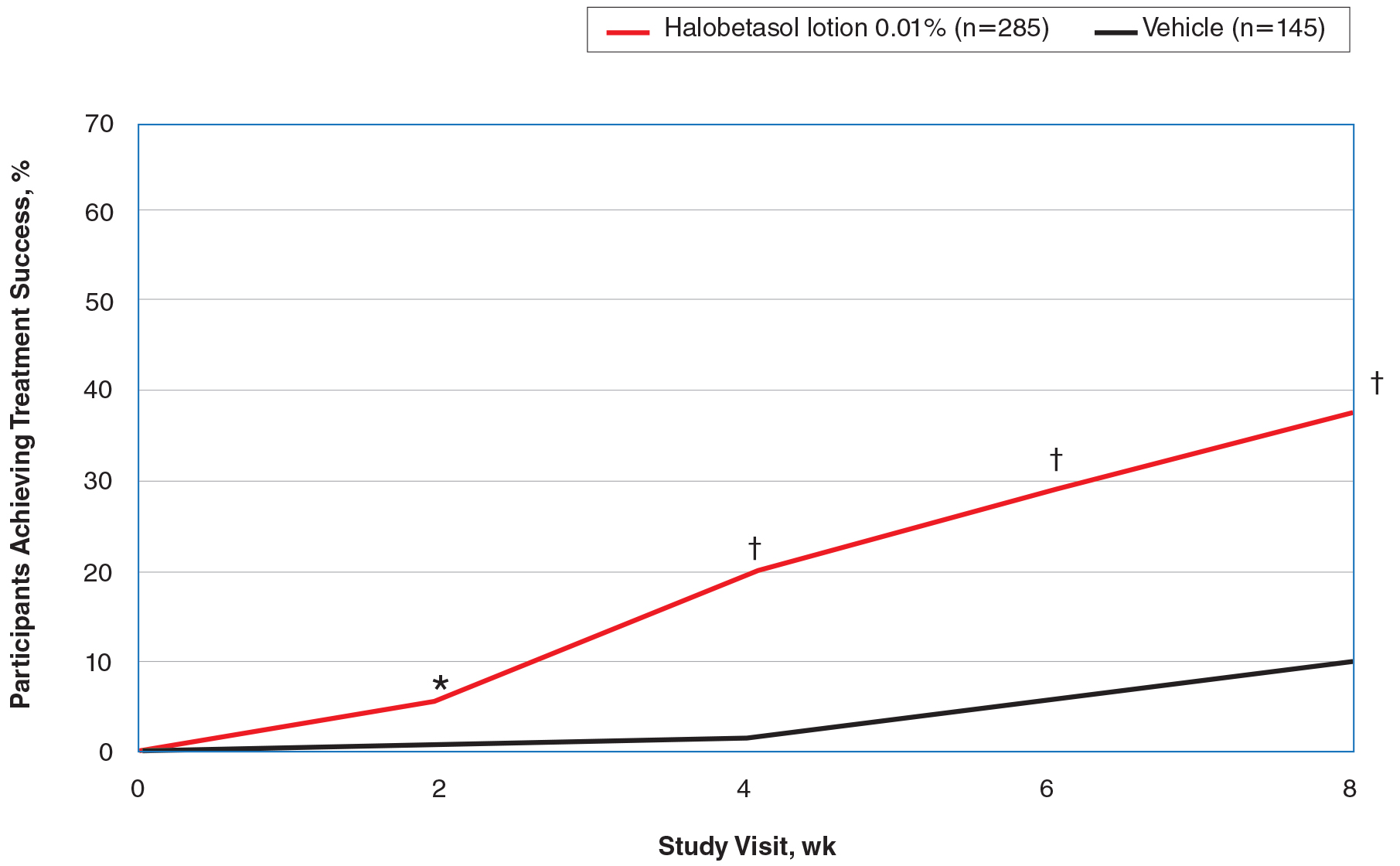
Figure 1. Investigator global assessment (IGA) of disease severity at each study visit: participants categorized as treatment successes (intention-totreat population pooled study data). Treatment success was defined as at least a 2-grade improvement in baseline IGA score and a score of 0 (clear) or 1 (almost clear). Asterisk indicates P=.003; dagger, P<.001.