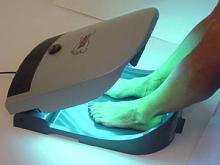
What's the Tootsie Tanner? A cleverly-named "portable" device aimed at people just plain embarrassed about fishy-looking feet. Who might that be? Essentially, it was marketed to golfers, tennis players, and others (teenagers, as always) concerned about, heaven forbid, VISIBLE SOCK LINES.
The $200 mini tanning bed, just for feet, was sold through the in-flight SkyMall magazine, on Amazon and via a variety of on-line retailers. Notice it "was" marketed. The FDA is reporting that after selling about 3,000 of these units, the manufacturer, IPCH, LLC is "out of business."
Not surprising, since the FDA sent a warning letter to the company about faulty labeling on its devices last year, and then again this spring. But that does mean that the Tootsie Tanner can't be serviced, replaced, or refunded at the company's expense, said the FDA.
So will golfers be forced to use sunless tanners or hit the links barefoot if they can’t find a salon or friend who's got an original Tootsie Tanner? There's always the $229 Solafeet Foot Tanner, which, "meets FDA standards."
Hey, FDA: One step forward, one step back.
-- Alicia Ault (on Twitter @aliciaault)