Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
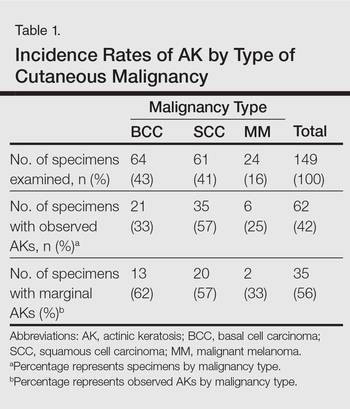
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).
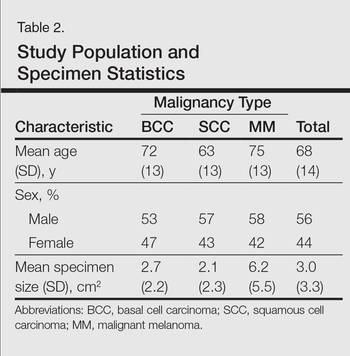
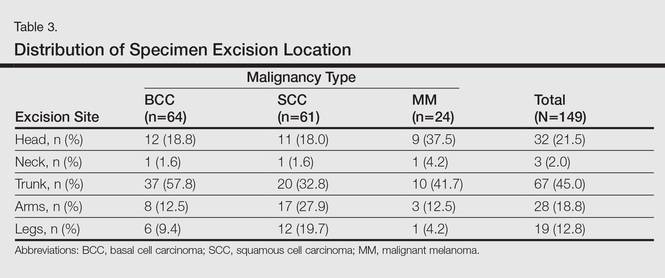
Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.
A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.