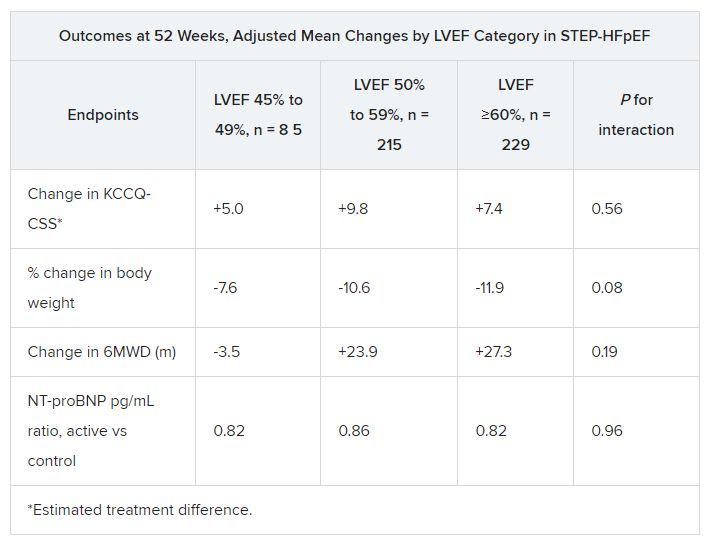
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.