Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
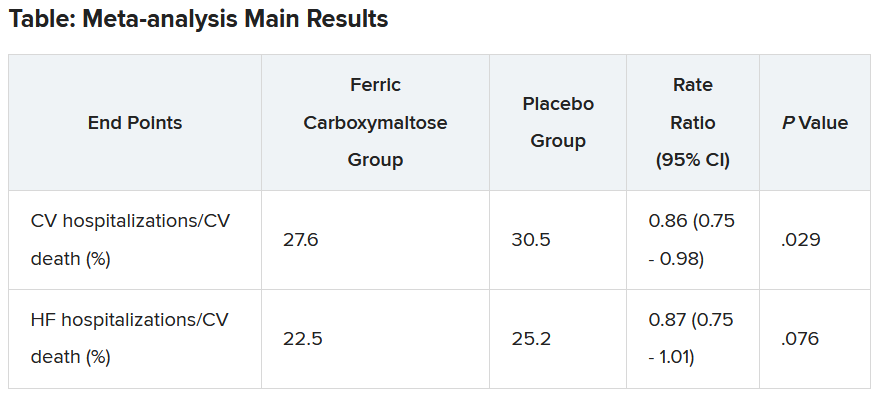
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.