Basis: An international study of 676 patients with previously treated unresectable stage III or IV melanoma whose disease had progressed, comparing ipilimumab plus an experimental tumor vaccine (gp100), gp100 alone, and ipilimumab alone. Median overall survival was 10 months among those who received ipilimumab with or without the vaccine, vs. 6 months among those who received the vaccine alone.
Addendum: Approved with a Risk Evaluation and Mitigation Strategy (REMS) program addressing life-threatening toxicity and deaths associated with ipilimumab in the study. Acts slowly, and some patients may have tumor progression before therapy becomes effective.
• Ruxolitinib (Jakafi oral tablets, Incyte Corp.). A Janus-associated kinase (JAK) inhibitor for the treatment of intermediate- or high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis. An orphan drug that is the first treatment approved for the rare blood disease – and the first approved JAK inhibitor.
Basis: Two randomized controlled trials of 528 patients with intermediate- or high-risk myelofibrosis. In one study, 42% of those on ruxolitinib had more than at least a 35% reduction in spleen volume vs. 1% of those on placebo at 24 weeks, and nearly half of those on treatment had achieved at least a 50% reduction in myelofibrosis symptoms, vs. 5% of those on placebo. In the second study, 29% of those on ruxolitinib had at least a 35% reduction in spleen size, vs. none of those on best available therapy after 48 weeks.
• Vandetanib (Caprelsa, AstraZeneca Pharmaceuticals LP). A kinase inhibitor for the treatment of symptomatic or progressive medullary thyroid cancer in adults with unresectable, locally advanced, or metastatic disease. The first drug approved for this rare cancer.
Basis: An international study of 331 patients with unresectable locally advanced or metastatic medullary thyroid cancer. Median progression-free survival was at least 22.6 months with vandetanib vs. 16.4 months among with placebo, a highly significant difference.
Addendum: Approved with a REMS addressing the risk of QT interval prolongation and a note that use in patients with indolent, asymptomatic, or slowly progressing disease "should be carefully considered" because of treatment-associated risks, as well as a boxed warning and a restricted distribution program.
• Vemurafenib (Zelboraf tablets, Hoffmann-LaRoche, Inc.). A BRAF inhibitor for the treatment of patients with unresectable or metastatic melanoma with the BRAFV600E mutation, as detected by the cobas 4800 BRAF V600 Mutation Test (Roche Molecular Systems), a companion diagnostic test approved at the same time. The second therapy found to prolong survival in patients with melanoma.
Basis: A study of 675 treatment-naive patients with unresectable or metastatic melanoma, positive for the BRAFV600E mutation. At the time of review, median survival was 7.9 months in a control group treated with dacarbazine, but had not been reached among those on vemurafenib. Median progression-free survival was 1.6 and 5.3 months, respectively.
Addendum: About half of melanoma patients have the BRAF mutation, and about half of them respond to vemurafenib. Approved with a medication guide to inform patients about the need for the diagnostic test and potential risks associated with treatment, including cutaneous squamous cell carcinoma. Roche and ipilimumab manufacturer Bristol-Meyers Squibb announced collaboration on a phase I/II study to determine whether combining the two agents is safe and effective in patients with BRAF mutations.
NEW DEVICES
• MelaFind (Mela Sciences). A noninvasive melanoma detection device for use "on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma."
Basis: The road to approval was controversial, with a split vote from an advisory panel, a citizens petition submitted by the manufacturer, and an agreement between the agency and manufacturer on how the device would be used and by whom.
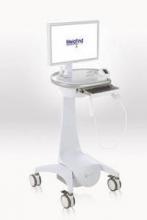
• NovoTTF-100A System (NovoTTF, Novocure). A portable, battery-powered device that delivers electrical fields to the brain as a treatment for adults with glioblastoma multiforme that has recurred after chemotherapy.
Basis: In a randomized study of 237 patients whose GBM had recurred after surgery, radiation, and chemotherapy, median overall survival was 6.3 months with the approximately 6-pound device and 6.4 months with best available chemotherapy. The agency cited evidence suggesting better quality of life without chemotherapy side effects.
NEW INDICATIONS
• Cetuximab (Erbitux, ImClone LLC, a wholly owned subsidiary of Eli Lilly & Co. and Bristol-Myers Squibb). The epidermal growth factor receptor (EGFR) antagonist in combination with platinum-based therapy plus 5-fluorouracil for the first-line treatment of recurrent locoregional and/or metastatic squamous cell carcinoma of the head and neck.