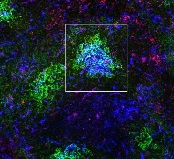
the spleen of a mouse, showing
inactivated GSK3 (magenta)
in B cells (blue) near follicular
dendritic cells (green).
Image from the lab of
Robert Rickert, PhD
Research published in Nature Immunology helps explain how B-cell metabolism adapts to different environments.
The study suggests the protein GSK3 acts as a metabolic checkpoint regulator in B cells, promoting the survival of circulating B cells while limiting the growth and proliferation of B cells in germinal centers.
“Our research shows that the protein GSK3 plays a crucial role in helping B cells meet the energy needs of their distinct states,” said study author Robert Rickert, PhD, of Sanford Burnham Prebys Medical Discovery Institute in La Jolla, California.
“The findings are particularly relevant for certain B-cell pathologies, including lymphoma subtypes, where there is an increased demand for energy to support the hyperproliferation of cells in a microenvironment that may be limited in nutrients.”
Dr Rickert and his colleagues noted that B cells predominate in a quiescent state until they encounter an antigen, which prompts the cells to grow, proliferate, and differentiate.
The team’s new study showed that GSK3 adjusts B-cell metabolism to match the needs of these different cell states.
In circulating B cells, GSK3 limits overall metabolic activity. In proliferating B cells in germinal centers, GSK3 slows glycolysis and the production of mitochondria.
In fact, GSK3 function is essential for B-cell survival in germinal centers. To understand why, the researchers looked at how B cells in these regions generate energy.
The team found that because these B cells are so metabolically active, they consume nearly all available glucose. That switches on glycolysis.
High glycolytic activity leads to an accumulation of toxic reactive oxygen species, as does rapid manufacture of mitochondria, which tend to leak the same chemicals.
Thus, by restraining the metabolism in specific ways, GSK3 prevents cell death induced by reactive oxygen species.
“Our results were really surprising,” Dr Rickert said. “Until now, we would have thought that slowing metabolism would only be important for preventing B cells from becoming cancerous, which it indeed may be. These studies provide insight into the dynamic nature of B-cell metabolism that literally ‘fuels’ differentiation in the germinal center to produce an effective antibody response.”
“It’s not yet clear whether or how GSK3 might be a target for future therapies for B cell-related diseases, but this research opens a lot of doors for further studies. To start with, we plan to investigate how GSK3 is regulated in lymphoma and how that relates to changes in metabolism. That research could lead to new approaches to treating lymphoma.”
This research was performed in collaboration with scientists at Eli Lilly and the Lunenfeld-Tanenbaum Research Institute at the University of Toronto. Funding was provided by the National Institutes of Health, the Lilly Research Award Program, the Arthritis National Research Foundation, and the Canadian Institutes of Health Research.

